USP 43 Atracurium besylate injection manufacturing process
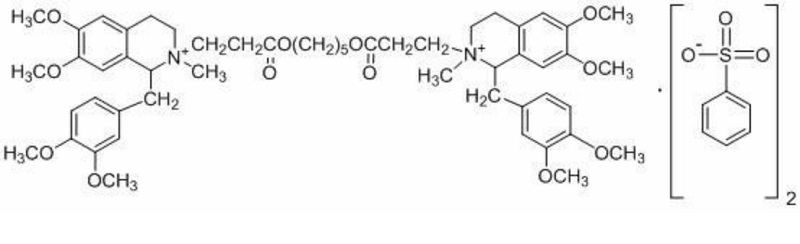
Atracurium besylate injection, molecular formula: C65H82N2O18S2, molecular weight: 1243.49, its structural formula is:
The indication is suitable for skeletal muscle relaxation during general anesthesia in various surgical operations, and also suitable for muscle relaxation required during tracheal intubation. It is a symmetrical double quaternary ammonium ester, a medium-aging non-depolarizing muscle relaxant with fast onset. As an anesthesia adjuvant, it is suitable for endotracheal intubation and chest and abdomen operations that require short-term muscle relaxation. Effective 1-2 minutes after intravenous injection, the muscle relaxation effect reaches the peak in 3-5 minutes, and the action time can be maintained for 15 minutes. The usual dose does not affect the heart, liver, and kidney functions, nor does it have obvious ganglion blocking effects, and does not produce bradycardia and other symptoms of vagus nerve excitement. The effect of histamine release is small. It is 1/3 of myostatin chloride, so the clinical dose has less chance of causing hypotension. High-dose rapid injection (1mg/kg) can cause tachycardia, hypotension caused by histamine release, and bronchospasm.
2,At present, there is no atracurium besylate injection in China, only atracurium besylate for injection. The FDA instructions indicate that the content of this product will decrease at a rate of 6% per year at 2-8°C, and the validity period is 2 year.
3, A preparation method for the scale-up production of atracurium besylate injection containing preservatives is provided. Aiming at the unstable characteristics of the product, through key process control, the product content is reduced at a rate of 2-8°C. Control within 4%, and on the basis of not increasing the feed, it can ensure that the content of the product remains above 90.0% within the two-year validity period.